Sat Jul 12 23:30:00 UTC 2025: Okay, here’s a summary of the text and a news article based on it:
**Summary:**
A new study published in *Immunity* reveals that fat metabolism within microglia, the brain’s immune cells, plays a crucial role in Alzheimer’s disease progression. Researchers at Purdue University, led by Gaurav Chopra, found that amyloid-beta plaques cause microglia to accumulate lipid droplets, impairing their ability to clear the plaques and protect neurons. This process involves the enzyme DGAT2, which converts fatty acids into fats stored in lipid droplets. Blocking DGAT2 in mice models reduced fat accumulation, restored microglia function, and significantly reduced plaque burden. Notably, female mice showed more severe microglial impairment compared to males, aligning with the higher risk of Alzheimer’s in women. While promising, experts caution that the animal model used may not perfectly reflect all forms and stages of the disease. Further research is needed, but this study offers a potential new therapeutic target for Alzheimer’s by manipulating lipid metabolism in the brain.
**News Article:**
**Indian Scientists Highlight New Research Showing Fat Metabolism in Brain’s Immune Cells Plays Key Role in Alzheimer’s**
**New Delhi, July 13, 2025** – Researchers are one step closer to understanding Alzheimer’s disease. A new study led by Purdue University researchers, with commentary from Indian Institute of Science professor Deepak Nair, suggests that managing fat metabolism in microglia could offer a new way to combat Alzheimer’s.
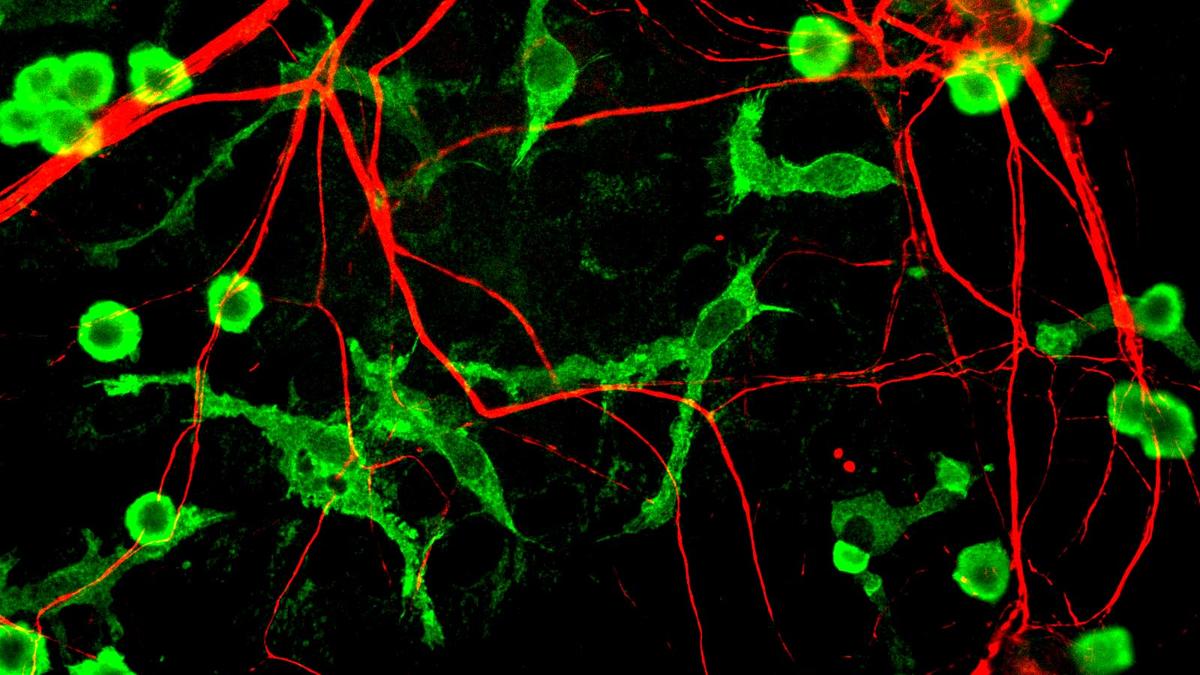
Alzheimer’s disease, a progressive brain disorder affecting millions worldwide, has long been linked to the accumulation of amyloid-beta plaques in the brain. Now, scientists are focusing on microglia, the brain’s immune cells, and their role in this process.
Published in the journal *Immunity*, the study details how amyloid-beta exposure triggers a buildup of fat within microglia, impairing their ability to clear the plaques and protect neurons. The research identifies the enzyme DGAT2 as a key player in this process, converting fatty acids into fats stored in lipid droplets within microglia.
“This study is pretty interesting and part of a growing body of studies indicating the role of fat metabolism problems in cells around amyloid plaques,” said Professor Nair.
Using mouse models of Alzheimer’s, researchers found that blocking DGAT2 reduced fat accumulation in microglia, restored their ability to clear amyloid plaques, and significantly reduced markers of neuronal damage. A one-week treatment in aged mice with advanced Alzheimer’s pathology drastically reduced plaque burden and neuronal damage markers.
“When we blocked DGAT2, we saw reduced fat accumulation in microglia and restoration of their ability to clear amyloid plaques. Even a one-week treatment in aged mice with heavy pathology drastically reduced the plaque burden by over 50% and significantly reduced neuronal damage markers,” Prakash said.
Notably, the study also revealed a sex-based difference, with female mice showing more severe microglial impairment than males, which aligns with the higher risk of Alzheimer’s in women.
While the study is promising, Professor Nair cautioned that the animal model used is an accelerated Alzheimer’s disease model that relies on Aβ pathology, so the findings may not be equally applicable to all forms or stages of the disease. He also emphasized that Alzheimer’s is a complex disease with multiple contributing factors beyond amyloid-beta.
“We’ve had over 100 drugs in clinical trials for Alzheimer’s in the past 20 years, and very few have succeeded. The disease is complex in its origin — it’s not caused by one thing,” said Prof. Nair. “If we can control just three or four critical pathways, lipid metabolism being one of them, it might be enough to slow down that collapse.”
The researchers are developing a microglia-specific degrader to target DGAT2, aiming to minimize potential side effects. While this is a long way away, Professor Nair believes that such efforts could slow the progression of the disease.
A five-year delay in the onset of Alzheimer’s could significantly reduce the socioeconomic burden of the disease, making these research efforts all the more critical.
